Rally urges health ministers to fund costly degenerative disease drug
Advertisement
Read this article for free:
or
Already have an account? Log in here »
To continue reading, please subscribe:
Monthly Digital Subscription
$0 for the first 4 weeks*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*No charge for 4 weeks then price increases to the regular rate of $19.95 plus GST every four weeks. Offer available to new and qualified returning subscribers only. Cancel any time.
Monthly Digital Subscription
$4.99/week*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*Billed as $19.95 plus GST every four weeks. Cancel any time.
To continue reading, please subscribe:
Add Free Press access to your Brandon Sun subscription for only an additional
$1 for the first 4 weeks*
*Your next subscription payment will increase by $1.00 and you will be charged $16.99 plus GST for four weeks. After four weeks, your payment will increase to $23.99 plus GST every four weeks.
Read unlimited articles for free today:
or
Already have an account? Log in here »
Hey there, time traveller!
This article was published 28/06/2018 (2751 days ago), so information in it may no longer be current.
Patients affected by a rare degenerative disease say there’s a potential cure within reach, but the federal and provincial governments aren’t prepared to fund a life-saving drug.
About 40 people affected by spinal muscular atrophy (SMA) came from as far as British Columbia and New Brunswick to rally Thursday at Winnipeg’s Fort Garry Hotel.
The downtown hotel is the site of a two-day annual summit between provincial and federal health ministers, where conversations are expected to run the gamut from mental health funding to cannabis framework, and the possible implementation of a national pharmacare plan.
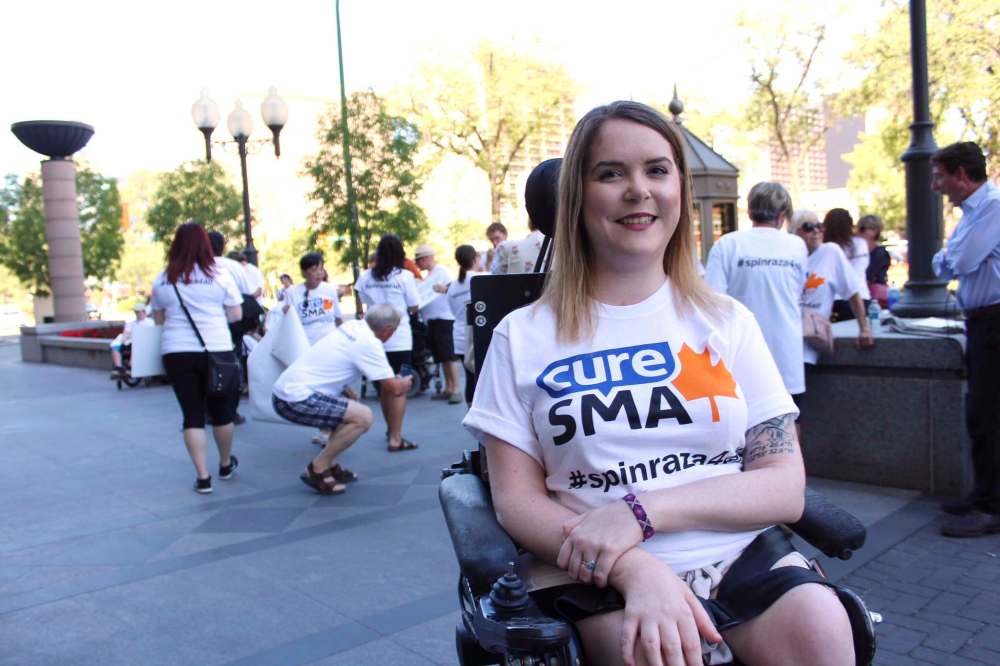
Susi Vanderwyk, executive director of Cure SMA Canada, said her group is hoping to get a few minutes with the ministers to explain its cause.
Cure SMA wants government to pay for a drug called Spinraza, which has been approved in 37 other countries and shown signs of helping slow symptoms of the muscle-wasting disease.
“What we know about the treatment is that it’s safe and that it’s effective, and that is from the clinical trial data, which is submitted to the government. And the patients who are taking it noticed a dramatic change,” said Vanderwyk, who has a 21-year-old daughter, Holli, living with SMA.
Some children in Canada with severe SMA have been allowed special access to Spinraza and shown signs of improvement, she noted.
Micaela Evans flew in from Vancouver to attend the rally. The 23-year-old has SMA, and hopes Canada will allow her access to Spinraza soon.
“I have a lot of friends in the U.S. who have taken (Spinraza) and they’ve been progressing, they’ve been able to gain a bit of strength. They’re able to move their heads more and even just (have) the stamina to get through your day a little longer… It makes a huge difference,” Evans said.
Life expectancy for those with SMA varies, but those with Type 1 don’t often live past their teenage years, said Durhane Wong-Rieger, president of the Canadian Organization for Rare Disorders. Those with SMA experience muscle deterioration and eventually lung failure, which leads to death, she said.
A spokesperson for the Manitoba government said they recognize the challenges faced by those with SMA and the importance of innovative therapies.
“Drug-funding decisions in Canada are made through a rigorous scientific review process where experts evaluate evidence of a drug’s benefits and cost effectiveness,” the spokesperson said.
“Discussions are ongoing between the pCPA (pan-Canadian Pharmaceutical Alliance) and drug manufacturer in regards to bringing Spinraza to Canadian patients at a fair price.”
Wong-Rieger said the government isn’t funding Spinraza due to its cost. According to a New York Times report in 2016, one dose of Spinraza carried a US$125,000 price tag, with patients requiring multiple doses annually for perhaps the rest of their lives.
Wong-Rieger estimated about 200 people live with SMA across Canada, and would require treatment.
“Of course, it’s a cost thing. It all comes down to the money. They’re claiming it’s to do with evidence, but frankly that’s a total cop-out. The evidence that was submitted here was the same evidence that was submitted to every other country,” she said.
“The longer they wait, the more (those with SMA) deteriorate – and you don’t recover function. You lose it, you lose it.”
jessica.botelho@freepress.mb.ca
Twitter: @_jessbu


