Ray of hope for cancer patients
Local firm's laser surgery approved by United States
Advertisement
Read this article for free:
or
Already have an account? Log in here »
To continue reading, please subscribe:
Monthly Digital Subscription
$0 for the first 4 weeks*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*No charge for 4 weeks then price increases to the regular rate of $19.00 plus GST every four weeks. Offer available to new and qualified returning subscribers only. Cancel any time.
Monthly Digital Subscription
$4.75/week*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*Billed as $19 plus GST every four weeks. Cancel any time.
To continue reading, please subscribe:
Add Free Press access to your Brandon Sun subscription for only an additional
$1 for the first 4 weeks*
*Your next subscription payment will increase by $1.00 and you will be charged $16.99 plus GST for four weeks. After four weeks, your payment will increase to $23.99 plus GST every four weeks.
Read unlimited articles for free today:
or
Already have an account? Log in here »
Hey there, time traveller!
This article was published 13/06/2009 (6005 days ago), so information in it may no longer be current.
A Winnipeg medical device company that has devised a way to blast inoperable brain tumours with laser treatment has received regulatory approval to market the technology in the United States.
After 10 years in development, Monteris Medical Inc. recently received clearance from the U.S. Food and Drug Administration that could mean hope for up to 90,000 patients a year who have been told their brain tumours are inoperable.
Company officials said it will seek Health Canada approval in 2010.

Conceived on the back of a napkin by researchers at St. Boniface General Hospital Research Centre in the late ’90s, clinical safety trials were successfully concluded recently on nine patients at the Cleveland Clinic and the University Hospitals Case Medical Center in Cleveland.
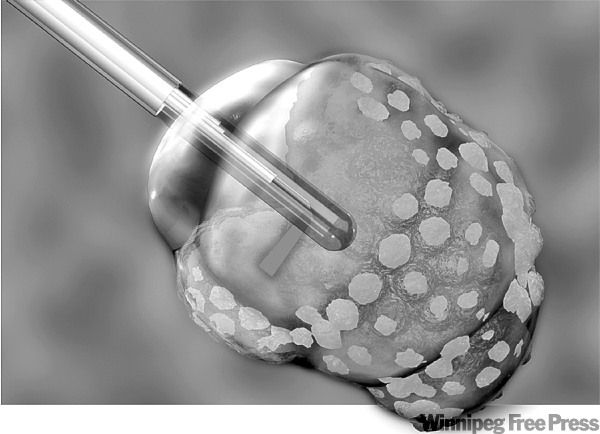
The technology, called the AutoLITT System, uses an MRI-guided laser probe, passed through a hole in the skull about the size of a dime, to deliver laser interstitial thermal therapy (LITT) to heat and coagulate the tumour from the inside.
Just like any brain surgery, there are risks involved, but company officials say the procedure could offer an option for some patients where previously none existed.
Dr. Mark Torchia, director of advanced technologies with the Winnipeg Regional Health Authority, and Richard Tyc, an engineer who is now director of engineering and technology at Monteris, worked on the technology from its infancy.
"Originally the thinking was if you can make a bur hole in the skull and insert a needle in it and do a biopsy, we wondered why we couldn’t treat the tumour with a laser," Tyc said. "That’s where it all started."
The technology "kiosk" includes a low-power MRI that allows objects to be brought into the field and provides image guidance to place the probe at exactly the right spot. Thermal-mapping with the MRI allows the surgeon to watch how far the heat from the end of laser is radiated so that healthy tissue is not affected.
"This is a huge milestone," Tyc said. "Now here we are, many years later with the ability to go to market."
The company has about 22 people at its Winnipeg offices in Smartpark and it has business development and sales people in the United States.

Brad Fercho, vice-president of business development, said the company expects to make its first sales this summer and Tyc said they hope to have 10 to 20 sites up and running next year. The AutoLITT technology is the firm’s sole product.
Harry Schulz, chief innovation officer for the Winnipeg Regional Health Authority, was the business development officer at St. Boniface General Hospital Research Centre in the late ’90s. He said it is not unusual to take more than 10 years to develop and commercialize such a medical device.
"If there was more critical mass (as far as investment capital is concerned) you might not have to pound the pavement for quite as long," Schulz said.
Over the years, the company raised about $15 million in venture capital including about $1.5 million from a Michigan economic development fund in 2008.
But its early-stage financing came from Winnipeg-based Western Life Sciences Venture Fund, Ensis Growth Fund (now GrowthWorks Canada) and the Business Development Bank.
"It is very difficult to get here," said Kevin McGarry, CEO of Western Life Sciences.
"It is a struggle. It always is. But this is wonderful, important technology."
Company officials wouldn’t estimate what the market potential is or the level of capital requirement the firm still requires to be able to continue to develop the technology and the market.
"The technology is proven now," McGarry said. "Now we have to prove the market. From all appearance the market could be very significant. But again, nothing sells itself."
Fercho said the company is well-funded but it is seeking additional capital to obtain the scale necessary to proceed with the commercialization.
"We expect to be cash-flow break even by 2011," he said. "Until then we rely on investors."
martin.cash@freepress.mb.
Monteris Medical Inc.
Ownership — privately owned, about 90 per cent Canadian. Largest shareholders are Western Life Science Venture Fund and Business Development Bank of Canada. The Southwest Michigan First Life Science Fund also owns a small stake.
CEO — James Duncan

Product — AutoLITT System, an MRI-guided laser probe
Cost per treatment — about $6,000
Winnipeg office — research and development and ongoing technology development
Kalamazoo, Mich. office — quality control and marketing

