Thousands of Manitobans affected by heart, blood-pressure drug recall
Read this article for free:
or
Already have an account? Log in here »
To continue reading, please subscribe:
Monthly Digital Subscription
$0 for the first 4 weeks*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*No charge for 4 weeks then price increases to the regular rate of $19.95 plus GST every four weeks. Offer available to new and qualified returning subscribers only. Cancel any time.
Monthly Digital Subscription
$4.99/week*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*Billed as $19.95 plus GST every four weeks. Cancel any time.
To continue reading, please subscribe:
Add Free Press access to your Brandon Sun subscription for only an additional
$1 for the first 4 weeks*
*Your next subscription payment will increase by $1.00 and you will be charged $16.99 plus GST for four weeks. After four weeks, your payment will increase to $23.99 plus GST every four weeks.
Read unlimited articles for free today:
or
Already have an account? Log in here »
Hey there, time traveller!
This article was published 16/07/2018 (2732 days ago), so information in it may no longer be current.
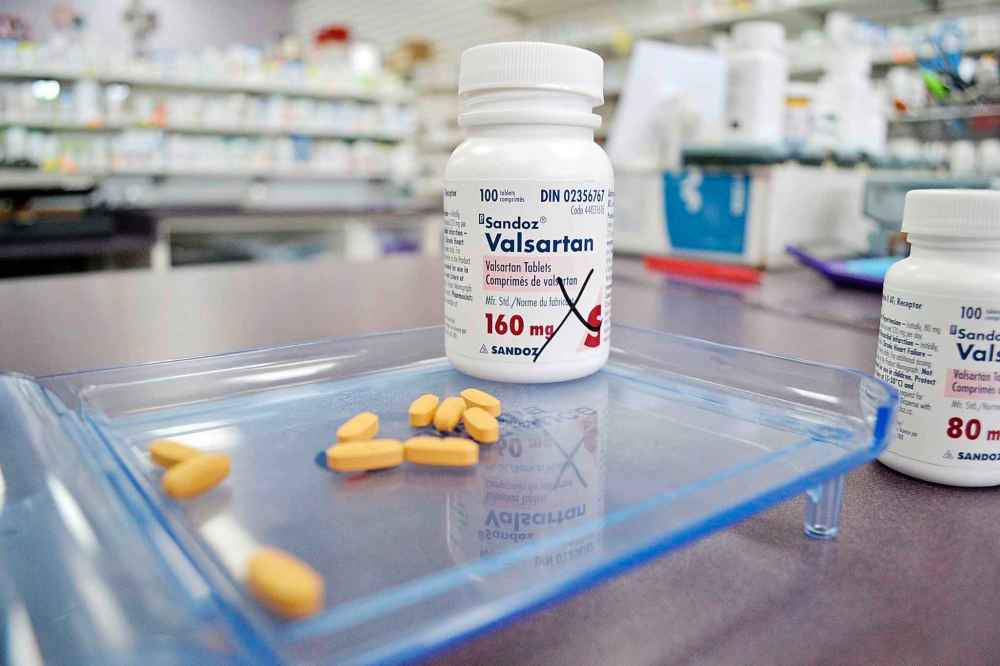
Thousands of Manitobans are taking heart and high blood pressure drugs with an impurity that could cause cancer.
But, even though Health Canada has recalled several varieties of valsartan, a generic drug, Manitobans and Canadians are being told not to stop taking them until a replacement drug is prescribed for them.
Barret Procyshyn, president of Pharmacists Manitoba, said Monday that there are about 10,000 Manitobans who currently have been prescribed drugs that contain valsartan.
Procyshyn said his organization is notifying all of its members about the recall and what to do.
“We’re dealing with it, but it’s a struggle,” he said. “There are up to 10,000 Manitobans on this medication. We’re dealing with it one person at a time… we can do everything except give it (alternative drug) out.”
Last week Health Canada announced it was pulling several drugs after it was determined that the valsartan manufacturer in China, which supplied the product to five drug companies to make the drugs, had the impurity N-nitrosodimethylamine in it — a potential human carcinogen.
Health Canada’s advisory
Information published on Health Canada’s website on July 9, 2018, including a list of affected medications.
Information published on Health Canada’s website on July 9, 2018, including a list of affected medications.
Issue
Several drugs containing the ingredient valsartan are being recalled by their manufacturers. An impurity, N-nitrosodimethylamine (NDMA), was found in the valsartan used in these products.
The valsartan was supplied by Zhejiang Huahai Pharmaceuticals. NDMA is a potential human carcinogen, which means that it could cause cancer with long-term exposure. Five companies have affected products, which are being recalled.
Drugs containing valsartan are used to treat patients with high blood pressure to help prevent heart attacks and stroke. These drugs are also used in patients who have had heart failure or a recent heart attack.
What you should do
Keep taking your medicine if it contains valsartan, unless you have been told to stop by your doctor or pharmacist.
If you are taking any medication containing valsartan, speak to your pharmacist who can tell you if your medicine is being recalled.
If you have been using an affected product, contact your health care practitioner as soon as possible to discuss your treatment options.
If you are in a clinical trial with a product containing valsartan and have any questions, speak to the doctor treating you in the trial.
Report side effects (adverse events) to health products to Health Canada by calling toll-free at 1-866-234-2345, or by reporting online, by mail or by fax.
Report complaints about health products to Health Canada by calling toll-free at 1-800-267-9675, or complete an online complaint form.
Twenty-two other countries have taken similar action.
Health Canada said the drug could cause cancer “with long-term exposure.”
Health Canada is telling people to keep using their drugs, even if they contain valsartan, “unless you have been told to stop by your doctor or pharmacist.”
As well, Health Canada is saying people taking the recalled product should “contact your health-care practitioner as soon as possible to discuss your treatment options.”
But Procyshyn said another worry is when thousands of Manitobans — and many more across the country — go back to a pharmacy to get their new medication.“There are up to 10,000 Manitobans on this medication. We’re dealing with it one person at a time… we can do everything except give it (alternative drug) out.”–Barret Procyshyn
“We hope the generic drug manufacturers can get enough of the alternatives,” he said. “There are going to be a lot of people switching in the next few weeks.

“This will cause pressure.”
Manitoba Health is also addressing another possible pressure — thousands of patients rushing to see their doctor in a short period of time.
A letter to physicians dated July 12 from Patricia Caetano, executive director of Manitoba’s provincial drug programs, said the drug recall “will result in a shortage of unknown duration and therefore, new starts of valsartan should be avoided, whenever possible.”
Caetano said because patients will likely first go to a pharmacy with questions before going to their doctor, “if there is a pharmacy that your patients most commonly use, please consider contacting them to develop a protocol for efficiently managing treatment changes as necessary.”
kevin.rollason@freepress.mb.ca

Kevin Rollason is a general assignment reporter at the Free Press. He graduated from Western University with a Masters of Journalism in 1985 and worked at the Winnipeg Sun until 1988, when he joined the Free Press. He has served as the Free Press’s city hall and law courts reporter and has won several awards, including a National Newspaper Award. Read more about Kevin.
Every piece of reporting Kevin produces is reviewed by an editing team before it is posted online or published in print — part of the Free Press‘s tradition, since 1872, of producing reliable independent journalism. Read more about Free Press’s history and mandate, and learn how our newsroom operates.
Our newsroom depends on a growing audience of readers to power our journalism. If you are not a paid reader, please consider becoming a subscriber.
Our newsroom depends on its audience of readers to power our journalism. Thank you for your support.








